Recent Publications
Does getting rid of tissue eosinophils help all people with EGIDs?
Benralizumab depletes eosinophils in EGID and improves symptoms
Reports on details of long term outcomes in those with EGID in the phase 2 trial of benralizumab. Shows that those with more of an allergic EGID phenotype had recurrent symptoms despite tissue depletion, while others had sustained clinical remission. Interestingly, review of pathology demonstrated continued epithelial tissue changes despite resolution of eosinophilia.
This is important because it suggests that certain EGIDS are completely resolved with targeted removal of eosinophils, while other forms, such as the allergic EGIDs, may not. This is one of the first studies showing divergence in disease pathogenesis within EGIDs.
Does getting rid of eosinophils in a rare disease (HES) help all patients?
Benralizumab Treatment for PDGFRA-negative Hypereosinophilic Syndrome
This phase 2 clinical trial demonstrated use of anti-eosinophil depletion therapy, benralizumab (anti-IL5Ra, to treat a rare disease, hypereosinophilic syndrome was safe and effective in a broad group of patients.
Importantly, it demonstrated complete tissue eosinophil depletion by a biologic. Interestingly, there were subjects who were complete non-responders to therapy despite having the IL5Ra target on their eosinophils. Click here to read the New England Journal of Medicine paper and find more about predictors of response.
Phase 3 clinical trials are pending (NCT04191304).
Is it hypereosinophilic syndrome (HES) or just EGID?
Single-organ and Multi-system HES with GI involvement
A common question allergists and gastroenterologists face when presented with patient with GI eosinophilia and blood eosinophlia, is whether this represents “just” EGID or should one be concerned with a multi-system hypereosinophilic syndrome (HES) with damage occurring in other organ systems. This retrospective study of NIH EGID patient cohorts systematically reviews clinical, endoscopic, and histopathological data to identify differences.
An important finding is that those with multi-system HES, one third had only GI symptoms at initial presentation, appearing to be just like EGID patients. This highlights the importance of monitoring for development of other end organ damage even in EGID patients.
What we have accomplished…
Eosinophils in Human Disease
Chhiba KD, Kuang FL, Kato, A, Berdnikovs S and Bochner B. “Little to no mRNA for the alpha or beta chains of FcεRI in human eosinophils” in J. Allergy Clin. Immunol.(2024) Feb;153(2):533-534 [PMC10922093]
Chhiba KD and Kuang FL “Advancing towards a unified eosinophil signature from transcriptional profiling” Invited review for Journal of Leukocyte Biology (2024) Aug 30:qiae188 online ahead of print [PMC11602342]
Hypereosinophilia and Hypereosinophilic Syndromes
Kuang FL, Legrand F, Makiya M, Ware J, Wetzler L, Brown T, Magee T, Piligian B, Yoon P, Hahn J, Sun X, Panch S, Powers A, Alao H, Kumar S, Quezado M, Yan L, Lee N, Kolbeck R, Newbold P, Goldman M, Fay MP, Khoury P, Maric I, and Klion AD. “Benralizumab treatment for PDGRA-negative hypereosinophilic syndromes” New England Journal of Medicine (2019) Apr 4;380(14):1336-46. [PMC6557265]
Kuang FL, Curtin BF, Alao H, Piligian B, Berry A, Holland-Thomas N, Powers A, Quezado M, Lumbard K, Fay M, Klion A, Kumar S, and Khoury P. “Single-organ and Multisystem hypereosinophilic syndrome (HES) patients with gastrointestinal manifestations share common characteristics” in J. Allergy Clin. Immunol. Pract.(2020) Apr 25:S2213-2198(20)30376-7 [PMC7483350]
Kuang FL, Sampaio De Melo M, Makiya M, Kumar S, Brown T, Wetzler L, Ware, J, Khoury P, Collins MH, Quezado M, Pittaluga S, and Klion AD. “Benralizumab completely depletes gastrointestinal tissue eosinophils and improves symptoms in eosinophilic GI disease.” J. Allergy Clin. Immunol. Pract(2022) Jun;10(6):1598-1605 [PMC9210216]
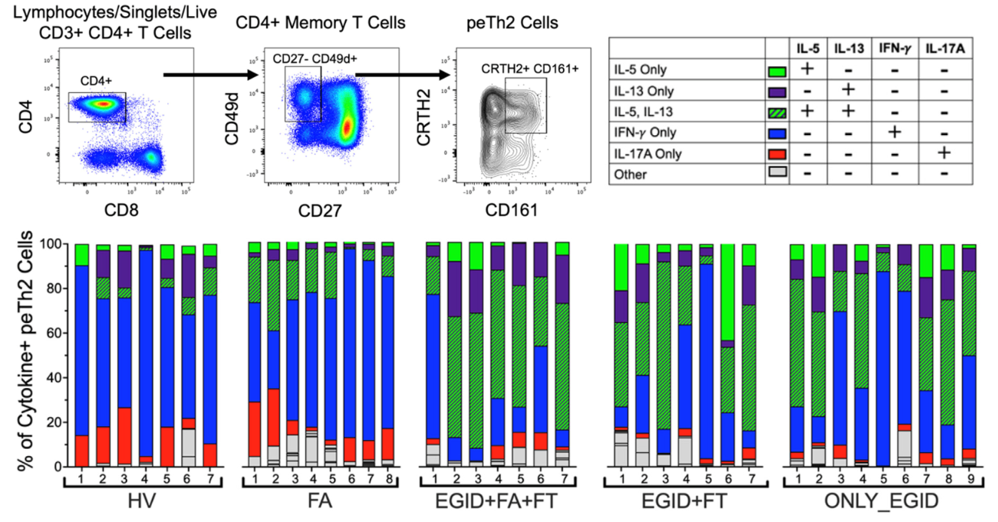
T cells in EGIDs
Makiya M, Brown T, Holland N, Wetzler L, Ware J, Khoury P, Frischmeyer-Guerrerio P, Klion AD and Kuang FL. “Unique cytokine profiles distinguish CRTH2+CD161+ (peTh2) T cells in patients with food allergy and eosinophilic gastrointestinal disorders.” – Clinical and Experimental Allergy (2023 Oct;53(10):1031-1040 [PMC10592354]
Janarthanam R, Kuang FL, Zalewski Z, Amsden K, Wang MY, Ostilla L, Keeley K, Hirano I, Kagalwalla A, Wershil B, Gonsalves N, and Wechsler JB. “Bulk T Cell Receptor sequencing confirms clonality in pediatric EoE and identifies a food-specific repertoire.” Allergy (2023) Sep;78(8):2487-96 [PMC10768854]
Reviews on Eosinophils and Eosinophil-Associated Diseases
Wright BL, Abonia JP, Aceves SS, Ackerman SJ, Brasket M, Chang J, Chehade M, Constantine G, Davis CM, Dellon ES, Doyle AD, Durbam R, Hill DA, Jensen ET, Kewalramani A, Khoury P, Klion AD, Kottyan L, Kuang FL, McGown EC, Ruffner MA, Spencer LA, Spergel J, Uchida A, Wechsler J, and Pesek RD. “Advances and Ongoing Challenges in Eosinophilic Gastrointestinal Disorders presented at the CEGIR/TIGERS Symposium at the 2024 American Academy of Allergy, Asthma & Immunology Meeting” in J. Allergy Clin. Immunol. (2024) Oct; 154(4):882-92. [PMC11456379]
Hirano I, Dellon ES, Falk GW, Gonsalves NP, Furuta GT, Bredenoord AJ; Kuang FL within ASCENT Working Group “Ascending to New Heights for Novel Therapeutics for Eosinophilic Esophagitis” Gastroenterology (2023), Sept 9:S0016-5083(23) [PMC10872872]
Kuang FL and Bochner BS “Lessons learned from targeting eosinophils in human disease” Seminars in Immunopathology (2021) Jun;43(3):459-475 [PMC8249333]
Kuang FL “Dexpramipexole: A Potential Non-biologic Alternative for Patients with Eosinophilic Asthma” TouchREVIEWS in Respiratory and Pulmonary Disease(2022) Volume 7(2) 36-38.
Kuang FL, Khoury P, Weller PF, Wechsler ME, and Klion AD “Biologics and hypereosinophilic syndromes: knowledge gaps and controversies” Invited Clinical Commentary J. Allergy Clin. Immunol. Pract. 2023 Sept; 11(9)2666-71. [PMC10527987]
Khoury P*, Roufosse F*, Kuang FL, Ackerman SA, Akuthota P, Bochner B, Johansson MW, Mathur SK, Ogbogu PU, Spencer LA, Wechsler ME, Zimmermann N, and Klion AD on behalf of the IES Clinical Research Interest Group “Biologic Therapy in Rare Eosinophil-Associated Disorders: Remaining Questions and Translational Research Opportunities” invited review for Journal of Leukocyte Biology (2024) Jul 25;116(2):307-320 [PMC11271980]
![Figure from Publication - “Advancing towards a unified eosinophil signature from transcriptional profiling” Invited review for Journal of Leukocyte Biology (2024) Aug 30:qiae188 online ahead of print [PMCID pending]](https://images.squarespace-cdn.com/content/v1/66a40822384c0d32d7e7bcb5/df55582b-de07-4c57-85ce-4d6ccf0fb8d6/Krishan+and+Fei+Li+JLB+Transcriptomics+paper.png)